SDG9
Professor Chung's Interdisciplinary Team from NCKU, Significantly Enhances Lithium-sulfur Battery Electrodes Performance
National Cheng Kung University's Distinguished Professor Chung-Chan Hung (Scopus) from the Department of Civil Engineering and Assistant Professor Sheng-Heng Chung (Scopus) from the Department of Materials Science and Engineering, after a cross-disciplinary collaboration, successfully stabilized the liquid polysulfides generated in lithium-sulfur batteries by using cement as an additive for the positive electrode. This research has been published in the professional ceramic field journal Ceramics International. ( Full content )
Lithium-sulfur batteries have advantages such as ultra-high capacity and energy density. Since the development in 2009, related research has been highly regarded and considered the next trend in commercial batteries. However, due to unresolved stability issues, the batteries have not yet been widely commercialized. Several main issues affecting the stability of lithium-sulfur batteries include poor conductivity of sulfur resulting in lower actual capacity, severe volume changes during charge and discharge processes leading to electrode structure damage, and the dissolution of liquid polysulfides produced during battery reactions in the electrolyte causing capacity loss and electrolyte contamination.
To address the issues of lithium-sulfur batteries, Assistant Professor Sheng-Heng Chung from the Department of Materials Science and Engineering and his research team member Yu-Jun Wang, also the first author of this paper, discovered that adding cement to lithium-sulfur batteries can improve battery performance. With the assistance of Professor Chung-Chan Hung from the Department of Civil Engineering, they conducted further detailed research and experiments on this matter. They found that silicon and oxygen elements in cement can adsorb sulfur and lithium generated during the charge and discharge of batteries. Therefore, adding cement to lithium-sulfur battery electrodes can effectively suppress the loss of original sulfides, which leads to capacity loss and electrolyte pollution, thereby improving battery stability to some extent.
In order to be closer to practical applications and alleviate industry concerns about the commercialization of lithium-sulfur batteries, the team also specifically focused on engineering data testing for high-loading batteries. They developed high-loading sulfur electrodes with a high sulfur loading capacity of 8.64 mg cm-2 and a sulfur content of 60 wt%, and achieved the lowest electrolyte-to-sulfur ratio of 7–3 µL mg−1 in a sparse electrolyte battery system (lithium-sulfur battery commercial design plan: sulfur loading >5 mg cm−2 and electrolyte-to-sulfur ratio <5 μL mg−1). This battery system has a high capacity output of 768 mA∙h g−1 and 11.06 mA∙h cm−2, which is 3-6 times the required capacity for commercial vehicle batteries. In addition, the lithium-sulfur batteries developed by the team can endure 100 cycles of dynamic long-term cycling and maintain 90% capacity in a 1-month static self-discharge test.
Assistant Professor Chung expressed appreciation and gratitude for the active promotion and encouragement of cross-disciplinary cooperation projects by NCKU in recent years, which provided abundant resources for collaboration. It was through the university's cross-disciplinary cooperation projects that he had the opportunity to collaborate with Professor Hung from the Department of Civil Engineering. Assistant Professor Chung believes that cross-disciplinary collaboration can break through one's original mindset, transcend the limitations of the original framework, create more possibilities, and cultivate integration capabilities for information and resources.
Lithium-sulfur batteries have advantages such as ultra-high capacity and energy density. Since the development in 2009, related research has been highly regarded and considered the next trend in commercial batteries. However, due to unresolved stability issues, the batteries have not yet been widely commercialized. Several main issues affecting the stability of lithium-sulfur batteries include poor conductivity of sulfur resulting in lower actual capacity, severe volume changes during charge and discharge processes leading to electrode structure damage, and the dissolution of liquid polysulfides produced during battery reactions in the electrolyte causing capacity loss and electrolyte contamination.
To address the issues of lithium-sulfur batteries, Assistant Professor Sheng-Heng Chung from the Department of Materials Science and Engineering and his research team member Yu-Jun Wang, also the first author of this paper, discovered that adding cement to lithium-sulfur batteries can improve battery performance. With the assistance of Professor Chung-Chan Hung from the Department of Civil Engineering, they conducted further detailed research and experiments on this matter. They found that silicon and oxygen elements in cement can adsorb sulfur and lithium generated during the charge and discharge of batteries. Therefore, adding cement to lithium-sulfur battery electrodes can effectively suppress the loss of original sulfides, which leads to capacity loss and electrolyte pollution, thereby improving battery stability to some extent.
In order to be closer to practical applications and alleviate industry concerns about the commercialization of lithium-sulfur batteries, the team also specifically focused on engineering data testing for high-loading batteries. They developed high-loading sulfur electrodes with a high sulfur loading capacity of 8.64 mg cm-2 and a sulfur content of 60 wt%, and achieved the lowest electrolyte-to-sulfur ratio of 7–3 µL mg−1 in a sparse electrolyte battery system (lithium-sulfur battery commercial design plan: sulfur loading >5 mg cm−2 and electrolyte-to-sulfur ratio <5 μL mg−1). This battery system has a high capacity output of 768 mA∙h g−1 and 11.06 mA∙h cm−2, which is 3-6 times the required capacity for commercial vehicle batteries. In addition, the lithium-sulfur batteries developed by the team can endure 100 cycles of dynamic long-term cycling and maintain 90% capacity in a 1-month static self-discharge test.
Assistant Professor Chung expressed appreciation and gratitude for the active promotion and encouragement of cross-disciplinary cooperation projects by NCKU in recent years, which provided abundant resources for collaboration. It was through the university's cross-disciplinary cooperation projects that he had the opportunity to collaborate with Professor Hung from the Department of Civil Engineering. Assistant Professor Chung believes that cross-disciplinary collaboration can break through one's original mindset, transcend the limitations of the original framework, create more possibilities, and cultivate integration capabilities for information and resources.

Assistant Professor Sheng-Heng Chung and his team, including Yu-Jun Wang, the paper's first author, found that adding cement to lithium-sulfur batteries enhances their performance.

Wang, the first author of this paper, conducted a demonstration of cement / multi-sulfide battery fabrication.
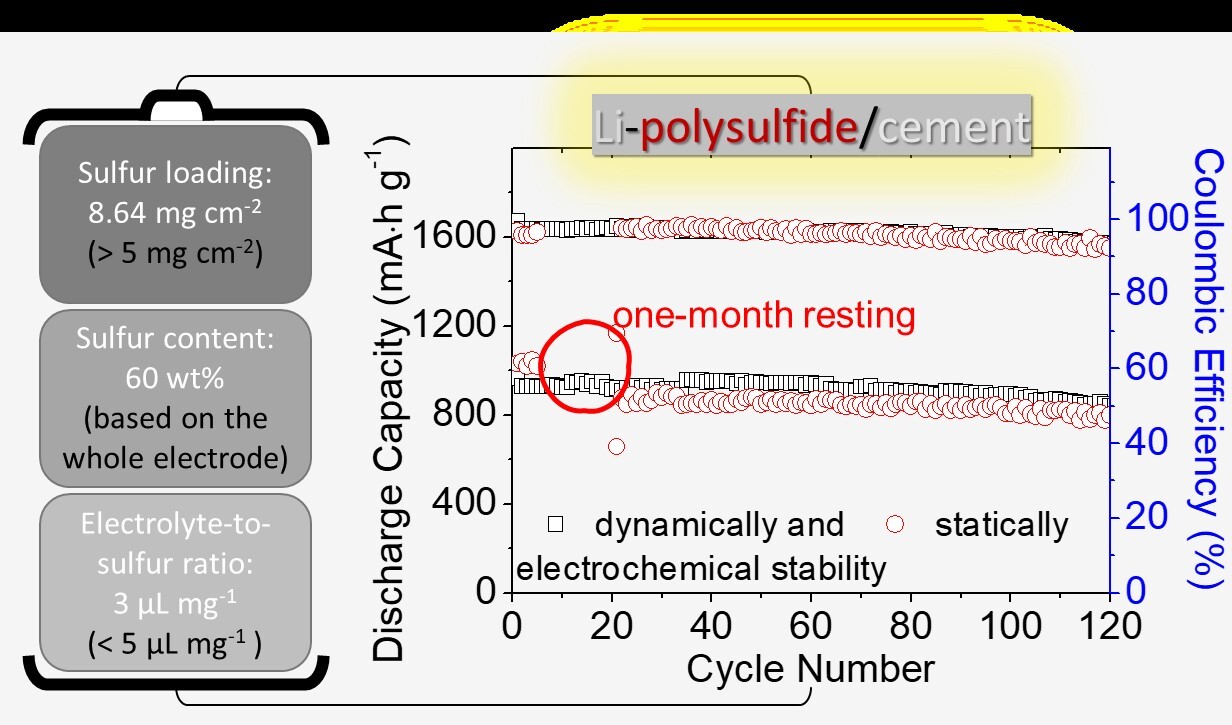
(left)high-loading, high-content, low-electrolyte integrated battery; (right) the dynamic long-cycle stability and static long-term low self-discharge performance of the battery.
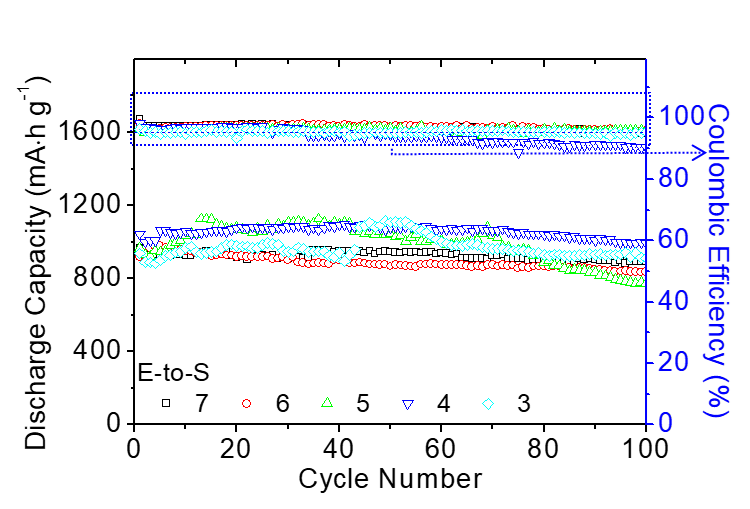
Battery Dynamic Long-Term Cycling Stability.
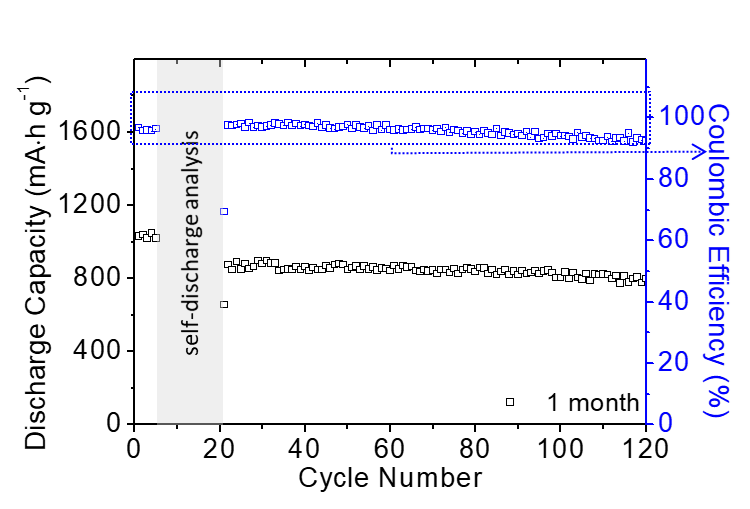
Battery Long-Term Static Low Self-Discharge Performance.

High-Load Electrode Integration in Low-Electrolyte Batteries.
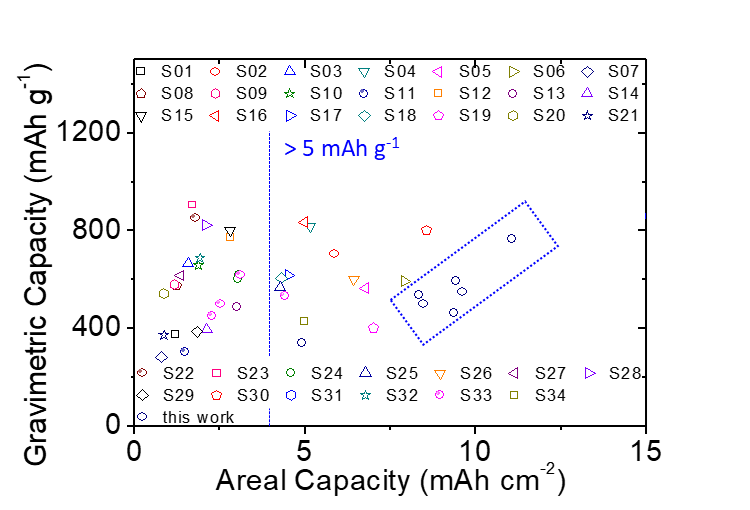
Battery High Practical Weight-to-Area Capacitance Ratio.























