RNA therapeutics have emerged as a promising field in the biomedical research domain. Professor Hung-Wei Yang's (Scopus) research team at the Department of Biomedical Engineering, NCKU, along with interdisciplinary collaborators, have utilized biotechnology to develop bacteriophage-based nanoparticles as carriers for delivering "interfering RNA" to target tumors. This approach aims to lower or suppress the expression of specific genes in tumors using RNA nanomedicines. Through animal experiments, the team successfully disrupted the self-repair mechanism of malignant brain cancer (glioblastoma) genes, thereby enhancing the effectiveness of radiation therapy. The research findings have been published in the ACS Nano journal under the title "Bioengineered Bacteriophage-Like Nanoparticles as RNAi Therapeutics to Enhance Radiotherapy against Glioblastomas," and have been featured on the cover.
The brain is protected by the "blood-brain barrier," making it challenging for conventional drug molecules to penetrate. Moreover, glioblastomas possess strong genetic repair capabilities, leading to suboptimal brain cancer treatment outcomes. With advancements in biomedical technology, using nanoparticles as carriers to traverse the blood-brain barrier holds promise for improving treatment effectiveness.
Professor Yang explained that the team conducted experiments using mice as subjects. They employed the "convection-enhanced delivery" method to directly deliver nanoscale RNA therapeutics into malignant brain tumors, overcoming the blood-brain barrier. Results showed that the treatment reduced the expression of two genes, EGFR and IKKα, within the tumors, thereby inhibiting the transcription factor NF-κb involved in malignant brain tumor cell DNA repair, ultimately disrupting tumor cell DNA repair.
In addition, the team subjected the mice with malignant brain tumors treated with RNA nanomedicines to low-dose X-ray radiation, resulting in a median survival time exceeding 60 days. In contrast, mice with malignant brain tumors only subjected to X-ray radiation had a median survival of just 31 days. Comparatively, when combined with clinical chemotherapy drug carboplatin and X-ray radiation, the treated mice exhibited a 20% improvement in median survival with fewer side effects.
Traditional methods for producing RNA nanomedicines are complex, involving separate steps of artificial RNA synthesis, carrier design, and subsequent integration. This process is time-consuming, labor-intensive, expensive, and lacks stability.
Professor Yang's team adopted a modular RNA scaffold design approach to synthesize the required RNA structures, allowing for rapid modifications tailored to different disease treatment needs. Professor Yang likened the modular RNA scaffold to LEGO blocks, where each block has various connection points, enabling versatile designs. Leveraging genetic engineering techniques, the team developed a one-step method for cells to autonomously generate the necessary RNA and directly encapsulate it within bacteriophages, significantly enhancing RNA stability and efficiency while reducing costs. This innovation played a pivotal role in the research being featured as a cover story.
Professor Yang noted that they've established a digital platform for modular RNA scaffold design. By selecting target gene sequences based on disease characteristics, the most stable RNA sequences can be computed and generated in just a few minutes.
This interdisciplinary collaboration involves NCKU, Linkou Chang Gung Memorial Hospital, and National Sun Yat-sen University. The research team includes Dr. Hao-Han Pang, a postdoctoral researcher, Dr. Nan-Yi Lee, a postdoctoral researcher, and Ph.D. candidate Ying-Pei Hsu from NCKU, as well as Dr. Guo-Zhen Wei, Dr. Pin-Yuan Chen, and Dr. Chiung-Ying Huang from Linkou Chang Gung Memorial Hospital.
Dr. Pang mentioned that the entire research spanned four years, with the most challenging aspect being the design of the modular RNA scaffold. Since there were no ready-made solutions available, the team had to develop and validate their own methods, even creating their own analysis techniques. From ground zero to gaining recognition in an international journal, the process was marked by numerous setbacks, and Dr. Pang expressed deep gratitude for the mutual support among team members. Professor Yang also extended his appreciation to cross-disciplinary collaborators such as Dr. Wei for their assistance in conducting animal experiments and verifying the possibilities in the animal experimentation phase.
"ACS Nano," published by the American Chemical Society, is a premier journal in the field of nanotechnology, nanomanufacturing, and nanoscience.
The brain is protected by the "blood-brain barrier," making it challenging for conventional drug molecules to penetrate. Moreover, glioblastomas possess strong genetic repair capabilities, leading to suboptimal brain cancer treatment outcomes. With advancements in biomedical technology, using nanoparticles as carriers to traverse the blood-brain barrier holds promise for improving treatment effectiveness.
Professor Yang explained that the team conducted experiments using mice as subjects. They employed the "convection-enhanced delivery" method to directly deliver nanoscale RNA therapeutics into malignant brain tumors, overcoming the blood-brain barrier. Results showed that the treatment reduced the expression of two genes, EGFR and IKKα, within the tumors, thereby inhibiting the transcription factor NF-κb involved in malignant brain tumor cell DNA repair, ultimately disrupting tumor cell DNA repair.
In addition, the team subjected the mice with malignant brain tumors treated with RNA nanomedicines to low-dose X-ray radiation, resulting in a median survival time exceeding 60 days. In contrast, mice with malignant brain tumors only subjected to X-ray radiation had a median survival of just 31 days. Comparatively, when combined with clinical chemotherapy drug carboplatin and X-ray radiation, the treated mice exhibited a 20% improvement in median survival with fewer side effects.
Traditional methods for producing RNA nanomedicines are complex, involving separate steps of artificial RNA synthesis, carrier design, and subsequent integration. This process is time-consuming, labor-intensive, expensive, and lacks stability.
Professor Yang's team adopted a modular RNA scaffold design approach to synthesize the required RNA structures, allowing for rapid modifications tailored to different disease treatment needs. Professor Yang likened the modular RNA scaffold to LEGO blocks, where each block has various connection points, enabling versatile designs. Leveraging genetic engineering techniques, the team developed a one-step method for cells to autonomously generate the necessary RNA and directly encapsulate it within bacteriophages, significantly enhancing RNA stability and efficiency while reducing costs. This innovation played a pivotal role in the research being featured as a cover story.
Professor Yang noted that they've established a digital platform for modular RNA scaffold design. By selecting target gene sequences based on disease characteristics, the most stable RNA sequences can be computed and generated in just a few minutes.
This interdisciplinary collaboration involves NCKU, Linkou Chang Gung Memorial Hospital, and National Sun Yat-sen University. The research team includes Dr. Hao-Han Pang, a postdoctoral researcher, Dr. Nan-Yi Lee, a postdoctoral researcher, and Ph.D. candidate Ying-Pei Hsu from NCKU, as well as Dr. Guo-Zhen Wei, Dr. Pin-Yuan Chen, and Dr. Chiung-Ying Huang from Linkou Chang Gung Memorial Hospital.
Dr. Pang mentioned that the entire research spanned four years, with the most challenging aspect being the design of the modular RNA scaffold. Since there were no ready-made solutions available, the team had to develop and validate their own methods, even creating their own analysis techniques. From ground zero to gaining recognition in an international journal, the process was marked by numerous setbacks, and Dr. Pang expressed deep gratitude for the mutual support among team members. Professor Yang also extended his appreciation to cross-disciplinary collaborators such as Dr. Wei for their assistance in conducting animal experiments and verifying the possibilities in the animal experimentation phase.
"ACS Nano," published by the American Chemical Society, is a premier journal in the field of nanotechnology, nanomanufacturing, and nanoscience.
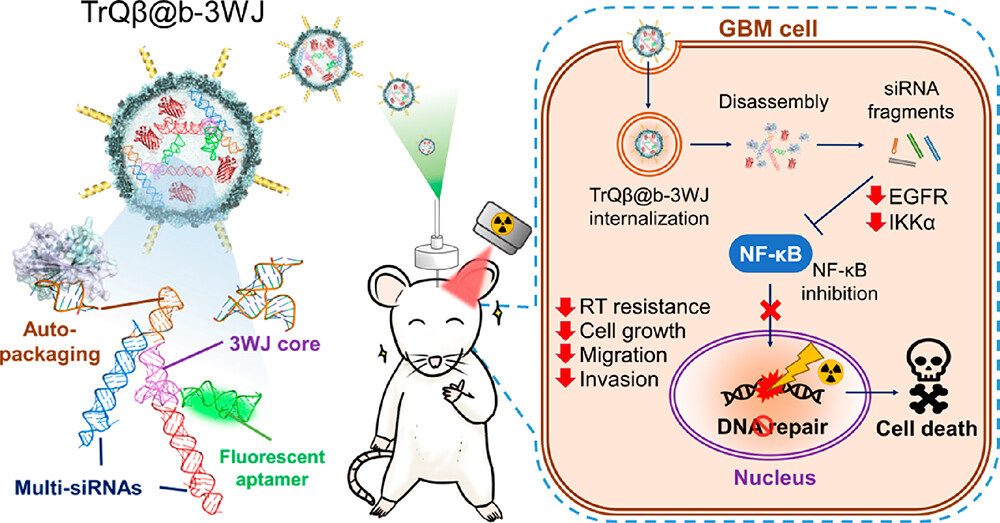
Using genetic engineering, RNA medication is injected intracranially to target tumor area, suppressing cancer cell genes.

Throughout the research period, overcoming numerous setbacks, finally achieving remarkable results and international recognition.

Through mutual support and diligence among interdisciplinary research team members, research achievements featured in a top international journal.

SDG3NCKU Soccer Team Claims 5th Place in National Division I — Best Record in Team History with Sports Science Support
View more
SDG3Chengda participates in the "ESG Vision for Good Circle", actively practicing university social responsibility
View more




















