SDG3
NCKU's Virtual Reality Mirror Therapy System receives FDA Class II medical device certification.
NCKU's Virtual Reality Mirror Therapy System (VRMT) has achieved FDA Class II medical device certification after years of research, clinical trials, and regulatory processes. It is the first VR-based rehabilitation software from a Taiwanese university to receive this certification (Product Code: QKC, Device Class: 2, Regulation Number: 890.5360).
VRMT combines motion perception with virtual reality to allow patients to see their healthy limb’s movements mirrored onto the affected side, enhancing neural plasticity and functional recovery. The software is compatible with Meta Oculus II and later versions and is available on Meta Store.
In rigorous clinical trials involving 54 patients, VRMT significantly outperformed traditional therapies, showing marked improvements in wrist movement and coordination. Results were published in the renowned journal Neurorehabilitation and Neural Repair.
Key team members include Professor Hsiu-Yun Hsu from NCKU Hospital's Department of Rehabilitation and Associate Professor Che-Wei Lin from the Department of Medical Engineering. Supported by national research and talent development programs, and with help from NCKU’s Advanced Medical Device Technology Center, VRMT has received FDA registration.
Currently, the system is available in the U.S. under physician prescription and will be showcased at the BIO Asia and Aging Health Industry Expo. NCKU aims to obtain Taiwan's FDA certification and collaborate with local VR headset manufacturers to further its application in Taiwan’s rehabilitation field.
VRMT combines motion perception with virtual reality to allow patients to see their healthy limb’s movements mirrored onto the affected side, enhancing neural plasticity and functional recovery. The software is compatible with Meta Oculus II and later versions and is available on Meta Store.
In rigorous clinical trials involving 54 patients, VRMT significantly outperformed traditional therapies, showing marked improvements in wrist movement and coordination. Results were published in the renowned journal Neurorehabilitation and Neural Repair.
Key team members include Professor Hsiu-Yun Hsu from NCKU Hospital's Department of Rehabilitation and Associate Professor Che-Wei Lin from the Department of Medical Engineering. Supported by national research and talent development programs, and with help from NCKU’s Advanced Medical Device Technology Center, VRMT has received FDA registration.
Currently, the system is available in the U.S. under physician prescription and will be showcased at the BIO Asia and Aging Health Industry Expo. NCKU aims to obtain Taiwan's FDA certification and collaborate with local VR headset manufacturers to further its application in Taiwan’s rehabilitation field.
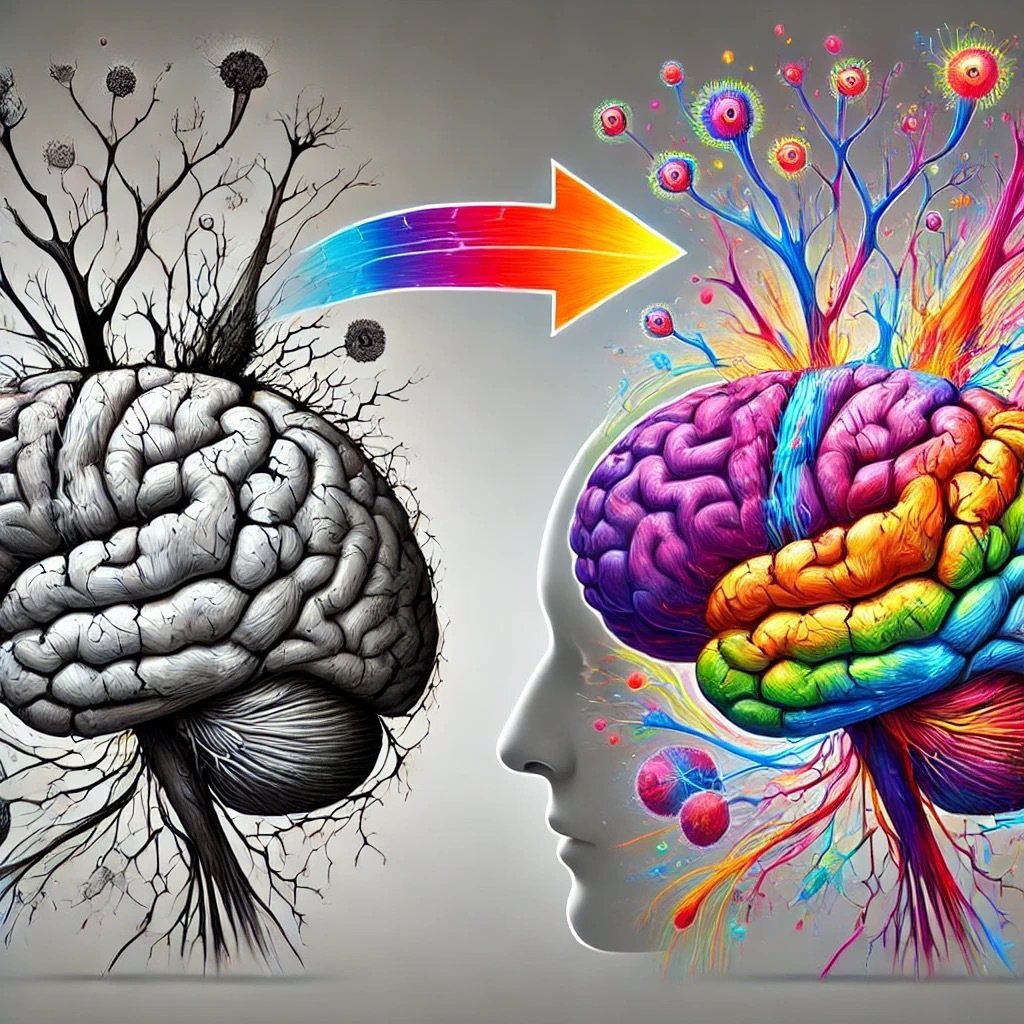
Mirror therapy with virtual reality combines motion perception and VR technology to allow patients to see their healthy limb's movements mirrored onto the affected limb in a virtual environment. This approach effectively stimulates the brain's neural plasticity.

NCKU's Virtual Reality Mirror Therapy (VRMT) system has successfully obtained FDA Class II medical device certification in the United States after years of research, development, clinical trials, and medical device application processes.
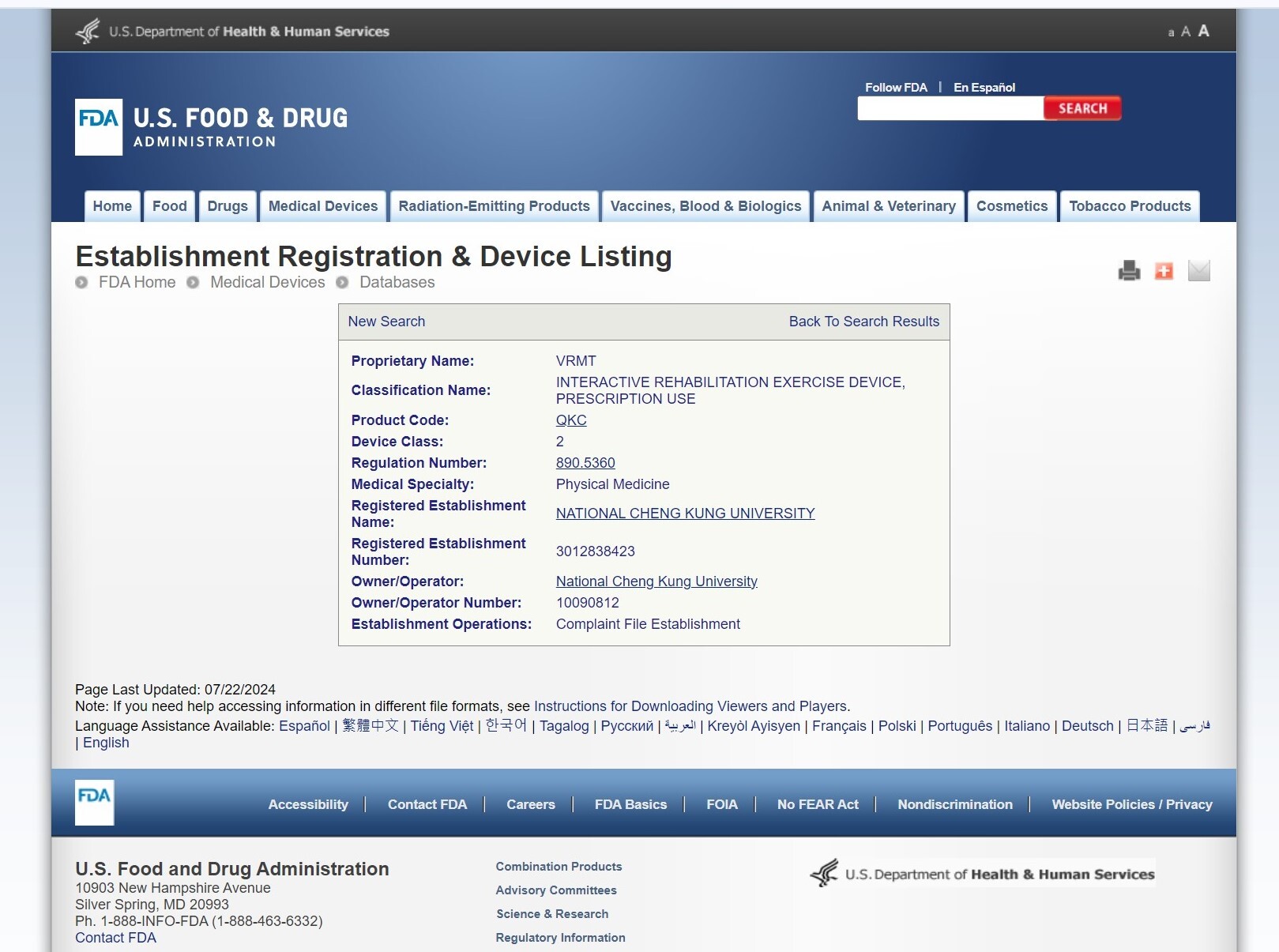
The U.S. FDA has published information on this product.






















