SDG3
NCKU Professor Chung’s team has developed “NCKU Digital Pathology Imaging Platform” for practical application
To enable research and development outcomes to be applied in the medical field, the Ministry of Health and Welfare's Food and Drug Administration (TFDA) has established stringent regulations. Professor Pau-Choo Chung's team at National Cheng Kung University (NCKU) obtained a medical device manufacturer license to ensure the "NCKU Digital Pathology Imaging Platform" could be legally adopted by hospitals. The team became the first in Taiwan to establish a comprehensive medical device software quality management system within a university and acquire a TFDA medical device license under this system. Confident in the clinical application of the "NCKU Digital Pathology Imaging Platform," the team plans to apply for U.S. FDA certification in 2025. Despite being a new challenge, the team is optimistic that success will facilitate global adoption.
Under Professor Pau-Choo Chung’s leadership, the team spent four years developing the "NCKU Digital Pathology Imaging Platform," which allows physicians to flexibly magnify and annotate pathology images on a computer. Recently, the platform received a TFDA medical device license and is set to be promoted for use in hospitals. Professor Pau-Choo Chung stated that the platform enables more precise analysis of pathology images, aiding clinical physicians in making accurate diagnoses and providing the most suitable medical solutions for patients.
Traditionally, pathology samples could only be observed under a microscope. The "NCKU Digital Pathology Imaging Platform" overcomes this limitation with flexible and user-friendly viewing capabilities, coupled with efficient annotation display technology. It effectively addresses the challenges of handling large-scale annotations in whole-slide digital pathology images. The platform also features ultra-high-resolution fast browsing, allowing physicians to seamlessly switch between different resolutions, query annotation results, and compare them with original images. All annotations and image storage formats comply with international standards, ensuring compatibility with other systems.
In addition to the digital pathology imaging platform, Professor Pau-Choo Chung’s team has actively developed "AI Digital Pathology Models" for various tumors over the years. The team plans to integrate these models with the platform to assist physicians in quickly examining and diagnosing tumor images. Currently, the AI models are undergoing the final stage of TFDA medical device registration.
Professor Pau-Choo Chung expressed gratitude to the medical team, TFDA, and the four major audit centers for medical device quality management systems for their support throughout the registration and inspection process. She emphasized the government’s commitment to fostering the medical device industry and NCKU’s dedication to advancing medical technology. Pathology AI modeling is a highly challenging but meaningful task. Professor Pau-Choo Chung hopes to inspire widespread collaboration in Taiwan, positioning the country as a global leader in digital pathology AI analysis.
Under Professor Pau-Choo Chung’s leadership, the team spent four years developing the "NCKU Digital Pathology Imaging Platform," which allows physicians to flexibly magnify and annotate pathology images on a computer. Recently, the platform received a TFDA medical device license and is set to be promoted for use in hospitals. Professor Pau-Choo Chung stated that the platform enables more precise analysis of pathology images, aiding clinical physicians in making accurate diagnoses and providing the most suitable medical solutions for patients.
Traditionally, pathology samples could only be observed under a microscope. The "NCKU Digital Pathology Imaging Platform" overcomes this limitation with flexible and user-friendly viewing capabilities, coupled with efficient annotation display technology. It effectively addresses the challenges of handling large-scale annotations in whole-slide digital pathology images. The platform also features ultra-high-resolution fast browsing, allowing physicians to seamlessly switch between different resolutions, query annotation results, and compare them with original images. All annotations and image storage formats comply with international standards, ensuring compatibility with other systems.
In addition to the digital pathology imaging platform, Professor Pau-Choo Chung’s team has actively developed "AI Digital Pathology Models" for various tumors over the years. The team plans to integrate these models with the platform to assist physicians in quickly examining and diagnosing tumor images. Currently, the AI models are undergoing the final stage of TFDA medical device registration.
Professor Pau-Choo Chung expressed gratitude to the medical team, TFDA, and the four major audit centers for medical device quality management systems for their support throughout the registration and inspection process. She emphasized the government’s commitment to fostering the medical device industry and NCKU’s dedication to advancing medical technology. Pathology AI modeling is a highly challenging but meaningful task. Professor Pau-Choo Chung hopes to inspire widespread collaboration in Taiwan, positioning the country as a global leader in digital pathology AI analysis.

Ministry of Health and Welfare Medical Device License
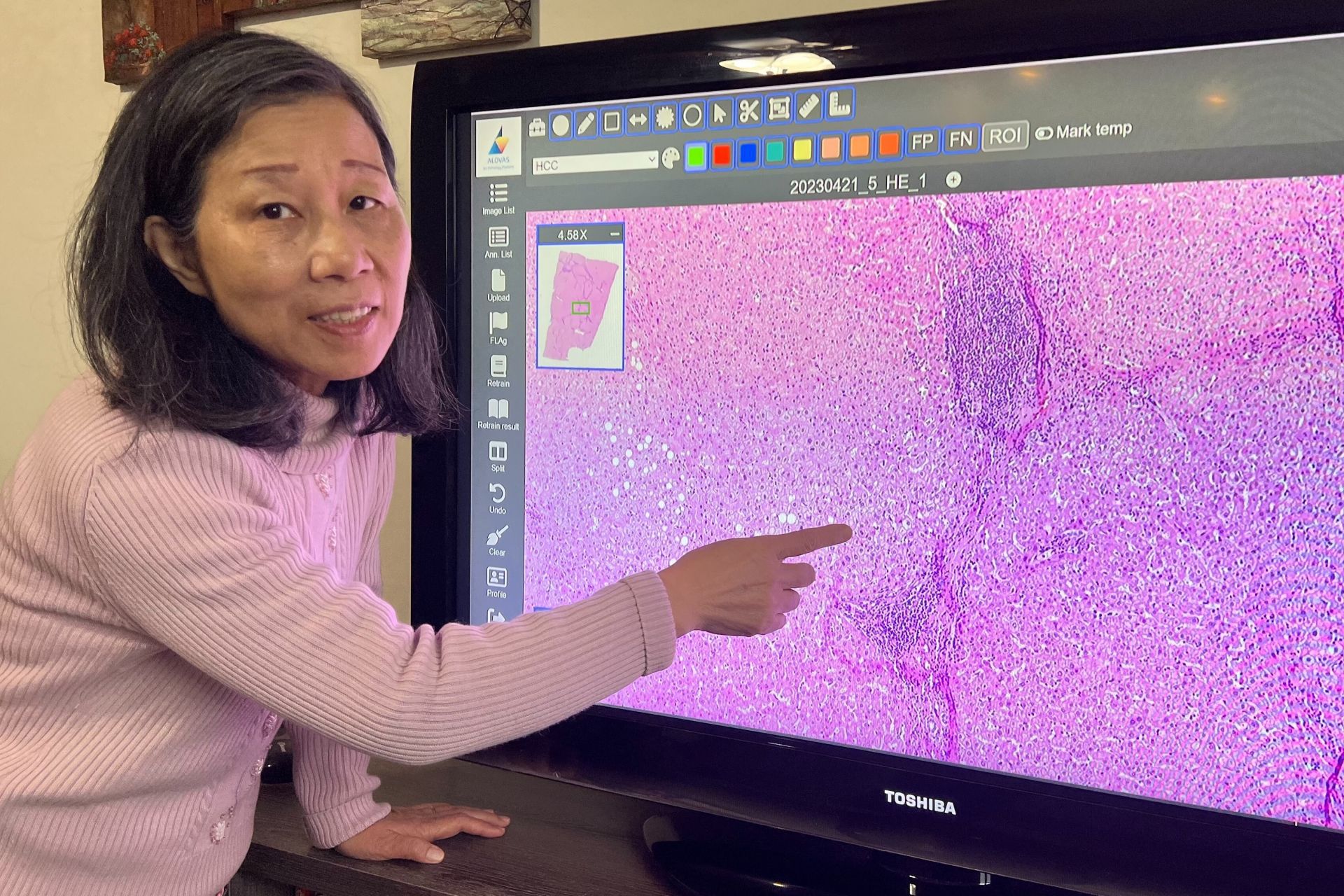
Professor Pau-Choo Chung reviews the images displayed on the “NCKU Digital Pathology Imaging Platform.”

Professor Pau-Choo Chung introduces the “NCKU Digital Pathology Imaging Platform” at the research exhibition.

SDG3"Medical Frontiers: NCKU Biomedical Research" Book Launch – Showcasing NCKU's Medical School Growth.
View more
SDG3NCKU Professor Yi-Ching Wang, dedicated to lung cancer for 27 years, receives Ministry of Education Academic Award.
View more



















