NCKU and NCYU Research Team Uncovers How Nanoplastics Disrupt Gut Microbiota – Featured in Nature Communications
Using mouse models, the team revealed a previously unknown mechanism by which nanoplastics impair gut health, marking a breakthrough in health risk studies related to plastic pollution. Within just three weeks of its online publication, the paper had already been read and downloaded nearly 20,000 times, was selected as a Highlight Article by the journal, and ranked in the top 1% of all contemporaneous publications globally in terms of altmetric score, reflecting widespread interest and discussion across academic and public platforms.
The team had previously uncovered related findings in a study on hydroponic vegetables. Though nanoplastics in water did not directly harm lettuce, they promoted the growth of specific bacteria in hydroponic systems. These bacteria released extracellular vesicles (EVs) that suppressed the plants' antioxidant systems and growth mechanisms, ultimately leading to plant death. This study, “Nanoplastics indirectly compromise lettuce growth in hydroponic systems via microbial extracellular vesicles derived from Curvibacter fontanus”, was published in the Journal of Hazardous Materials.
According to Professor Hsu, microplastics have emerged as a major environmental concern—polluting not only oceans and soil, but now also showing potential risks to crop health and the human body.
She explained that microplastics vary widely in size. While particles larger than 150 microns are often expelled from the body, nanoplastics (with 1 micron = 1,000 nanometers) may accumulate in vital organs. These ultra-small particles can even penetrate the gut and brain’s natural barriers, allowing inflammatory factors or pathogens to invade. Due to the difficulty in observing such processes, relevant studies have remained limited.
The research team emphasized that extracellular vesicles are currently a hot topic in biomedical research, especially in the context of cross-species communication (e.g., between microbes and animals). EVs are tiny vesicles secreted by cells, acting as messengers to deliver molecular signals (such as microRNAs) to other cells, thereby influencing their function.
In this study, mice were fed nanoplastics over a 12-week period. The team found that exosomes released from intestinal cells and specific gut bacteria, loaded with microRNAs, could reprogram the gut’s operational signals. This altered the host-microbiota relationship, reducing beneficial bacteria, increasing harmful ones, and damaging the gut’s natural protective barrier—ultimately inducing microbiota imbalance and contributing to intestinal permeability (leaky gut). The researchers emphasized that these two studies are the first to systematically reveal how nanoplastics can harm organisms indirectly via EVs, whether in plants or animals. The findings underscore the widespread risk and underestimated toxicity of nano-sized plastic pollution.
Professor Hsu emphasized that while the research decodes the molecular mechanism behind nanoplastics-induced dysbiosis, differences between human and mouse microbiota mean further studies are needed before implications for human health can be confirmed. To that end, the team has developed a biomimetic dynamic gut microbiota system to assess the effects of nanoplastics and other substances on the human gut microbiome. She added that gut inflammation and mucosal damage can create gaps between intestinal cells, allowing substances like undigested food, bacteria, and toxins to enter the bloodstream—leading to a range of health issues, the hallmark of leaky gut syndrome.
Professor Hsu noted that this research not only broadens our understanding of microplastic toxicity, but also highlights the central role of extracellular vesicles in host-microbiota communication. This opens new therapeutic directions—such as potentially regulating EV production or modifying microRNA signaling to counteract the effects of nanoplastics.
Assistant Professor Lee added that this study focuses on the novel and important role of EVs in mediating interactions between hosts and microbes, aiming to understand how they influence gut microbiota composition and host health—paving the way for EV-based therapeutic targets in the future.
The research was supported by Taiwan’s National Science and Technology Council (NSTC) “Young Scholar Fellowship” and the Ministry of Health and Welfare, demonstrating Taiwan’s strong capacity for scientific innovation. With extracellular vesicle-based technologies rapidly emerging worldwide, the research team hopes Taiwan will invest more in EV-related research and applications, enhancing global visibility and securing a leading position in this rapidly advancing field.

Associate Professor Wei-Hsuan Hsu from the Department of Food Safety / Hygiene and Risk Management at NCKU, and Assistant Professor Bao-Hong Lee from the Department of Horticulture at National Chiayi University, have discovered that nanoparticles can “hijack” the communication systems between gut cells and bacteria. This interference alters the symbiotic relationship between microbes and their host, not only disrupting the balance but also impairing the intestinal barrier function, potentially leading to leaky gut syndrome. This groundbreaking research has drawn significant international attention and was recently published in the prestigious journal Nature Communications.
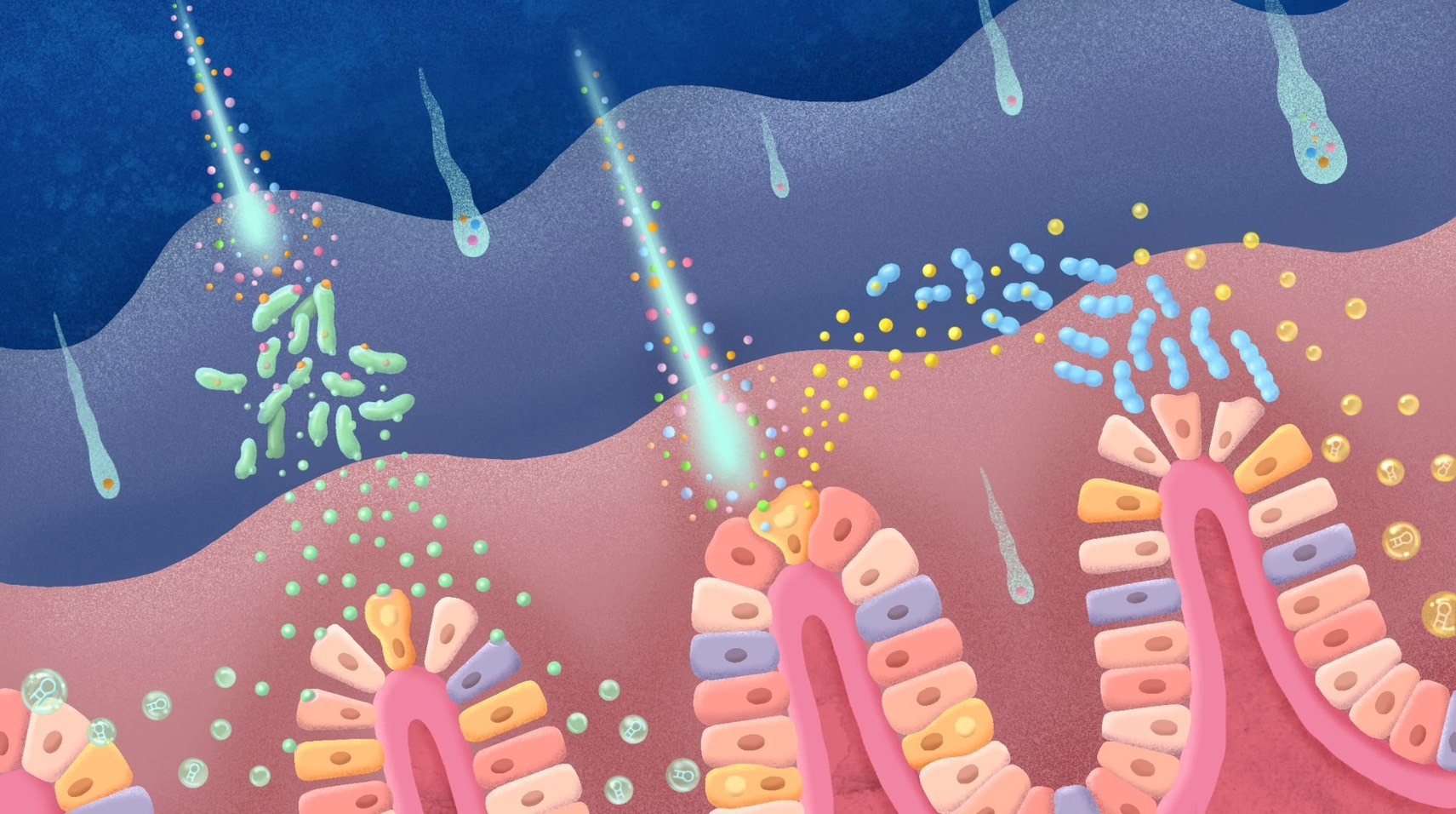
Foreign nanoplastics act like comets piercing through the intestinal protective barrier, attacking both gut bacteria and intestinal cells. In response to this stimulation, the bacteria and cells secrete extracellular vesicles (exosomes), which in turn affect the tight junctions between intestinal cells and the composition of the gut microbiota, ultimately leading to leaky gut syndrome.
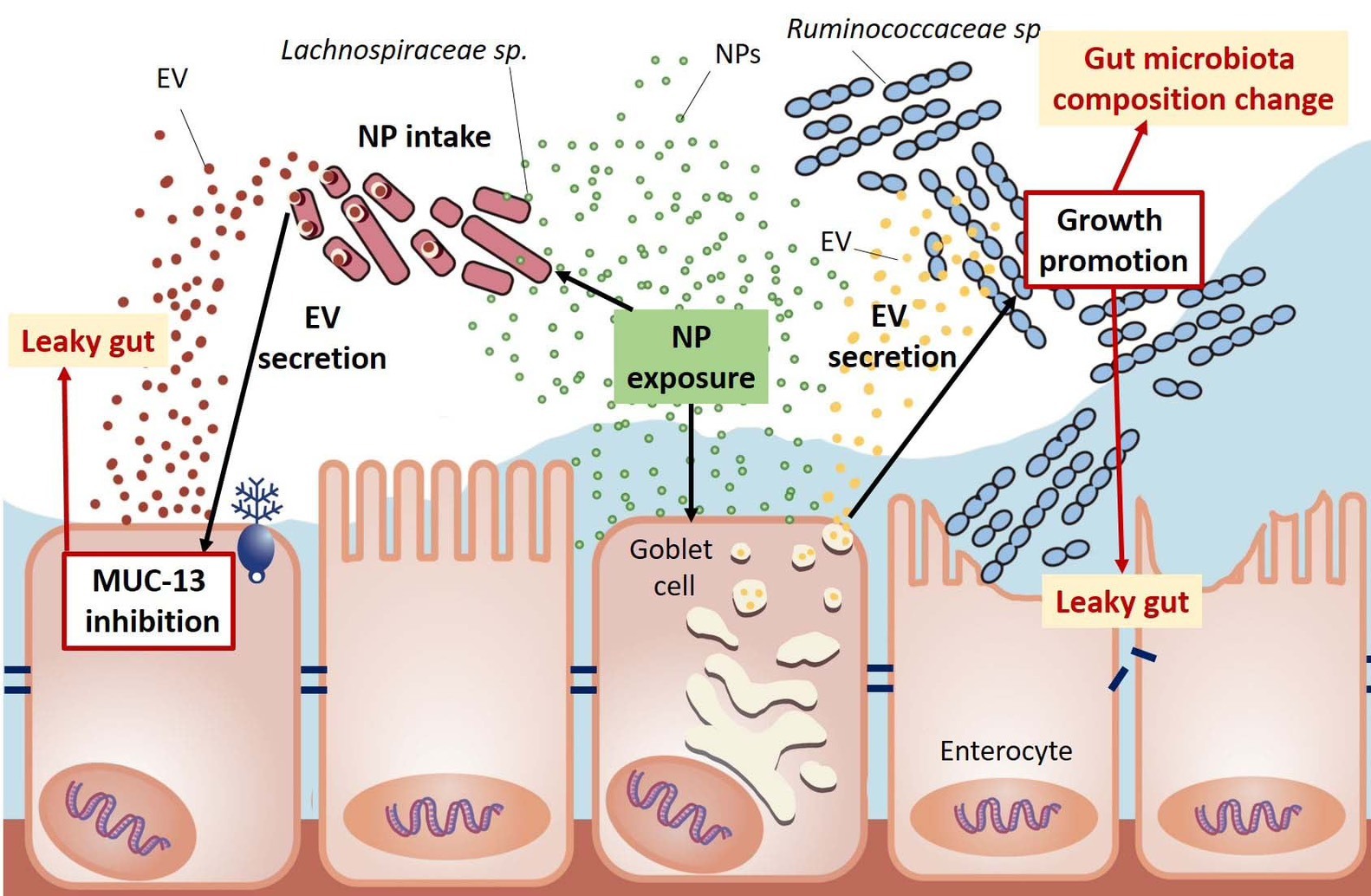
Nanoplastics disrupt the balance of the gut microbiota and impair intestinal barrier function by influencing the release of extracellular vesicles (exosomes) from gut bacteria and intestinal cells.

The joint research team from NCKU and NCYU has been dedicated to studying the functions of microbial extracellular vesicles (exosomes). Their achievements were recognized with the 2024 Future Tech Award from the National Science and Technology Council (NSTC). In the photo, fourth from the right is Associate Professor Wei-Hsuan Hsu from the Department of Food Safety / Hygiene and Risk Management at NCKU, and third from the left is Assistant Professor Bao-Hong Lee from the Department of Horticulture at NCYU, pictured with their lab students.






















