Written by Pin Ling.Image credit to NCKU News Center.
Even though the COVID-19 pandemic fades away, research efforts never stop gaining more insights into viral infections, like SARS-CoV-2 and influenza, for better preparation for the next pandemic. A recent study from Dr. Pin Ling and his team in the Department of Microbiology and Immunology at National Cheng Kung University (NCKU) provides a novel insight into the host’s first sense of viral infection to trigger the early antiviral defense. Retinoic acid-inducible gene I (RIG-I) is a cytosolic viral RNA sensor critical for host antiviral defense against RNA virus infection, including SARS-CoV-2 and influenza. RIG-I sensing of viral infection mainly occurs in the cytoplasm after virus invasion and replication within a cell. Distinct from this idea, Ling et al. noted that RIG-I is recruited onto endosomes, the “gateway” of virus invasion into a host cell, to detect viral RNA immediately upon breaching endosomes. Further, endosomes also serve as signaling platforms for RIG-I activation. Endosomal adaptor TAPE (TBK1-Associated Protein in Endolysosomes) is crucial for mediating the RIG-I complex formation and plays a key role in defending against influenza virus infection in vivo.
The host’s immune response timing is vital for the arms race between the host and viruses. This novel RIG-I action mode by Ling’s work allows the host to detect viral infection and induce antiviral immunity much earlier than the previous idea. Insights from this study may also help future vaccine design and antiviral and autoimmune disease treatment. This study entitled “Endosomes Serve as Signaling Platforms for RIG-I Ubiquitination and Activation” has been published in Science Advances, a prestigious journal under the Science group. Since its publication in early November 2024, it has been downloaded nearly 2,000 times within a short period (excluding views), garnering global attention.
This research spanned a decade and involved contributions from key collaborators, including Dr. Kuan-Ru Chen from the Department of Medical Research, E-Da Hospital; Dr. Tse-Hua Tan from the Immunology Research Center, National Health Research Institutes; Dr. Jia-Yu Yang from the Department of Microbiology and Immunology, Chang Gung University; Drs. Xu San-Ging Shu and Chao-Liang Wu from the Department of Pediatric, Ditmanson Medical Foundation Chia-Yi Christian Hospital. Dr. Ling also acknowledged other colleagues in the group and expressed his excitement about the team's success after overcoming numerous challenges and setbacks, emphasizing the importance of cross-disciplinary collaboration.
Provider: NCKU News Center
Date: 2024-12-05
Even though the COVID-19 pandemic fades away, research efforts never stop gaining more insights into viral infections, like SARS-CoV-2 and influenza, for better preparation for the next pandemic. A recent study from Dr. Pin Ling and his team in the Department of Microbiology and Immunology at National Cheng Kung University (NCKU) provides a novel insight into the host’s first sense of viral infection to trigger the early antiviral defense. Retinoic acid-inducible gene I (RIG-I) is a cytosolic viral RNA sensor critical for host antiviral defense against RNA virus infection, including SARS-CoV-2 and influenza. RIG-I sensing of viral infection mainly occurs in the cytoplasm after virus invasion and replication within a cell. Distinct from this idea, Ling et al. noted that RIG-I is recruited onto endosomes, the “gateway” of virus invasion into a host cell, to detect viral RNA immediately upon breaching endosomes. Further, endosomes also serve as signaling platforms for RIG-I activation. Endosomal adaptor TAPE (TBK1-Associated Protein in Endolysosomes) is crucial for mediating the RIG-I complex formation and plays a key role in defending against influenza virus infection in vivo.
The host’s immune response timing is vital for the arms race between the host and viruses. This novel RIG-I action mode by Ling’s work allows the host to detect viral infection and induce antiviral immunity much earlier than the previous idea. Insights from this study may also help future vaccine design and antiviral and autoimmune disease treatment. This study entitled “Endosomes Serve as Signaling Platforms for RIG-I Ubiquitination and Activation” has been published in Science Advances, a prestigious journal under the Science group. Since its publication in early November 2024, it has been downloaded nearly 2,000 times within a short period (excluding views), garnering global attention.
This research spanned a decade and involved contributions from key collaborators, including Dr. Kuan-Ru Chen from the Department of Medical Research, E-Da Hospital; Dr. Tse-Hua Tan from the Immunology Research Center, National Health Research Institutes; Dr. Jia-Yu Yang from the Department of Microbiology and Immunology, Chang Gung University; Drs. Xu San-Ging Shu and Chao-Liang Wu from the Department of Pediatric, Ditmanson Medical Foundation Chia-Yi Christian Hospital. Dr. Ling also acknowledged other colleagues in the group and expressed his excitement about the team's success after overcoming numerous challenges and setbacks, emphasizing the importance of cross-disciplinary collaboration.
Provider: NCKU News Center
Date: 2024-12-05

Dr. Pin Ling and his team in the Department of Microbiology and Immunology at National Cheng Kung University (NCKU)
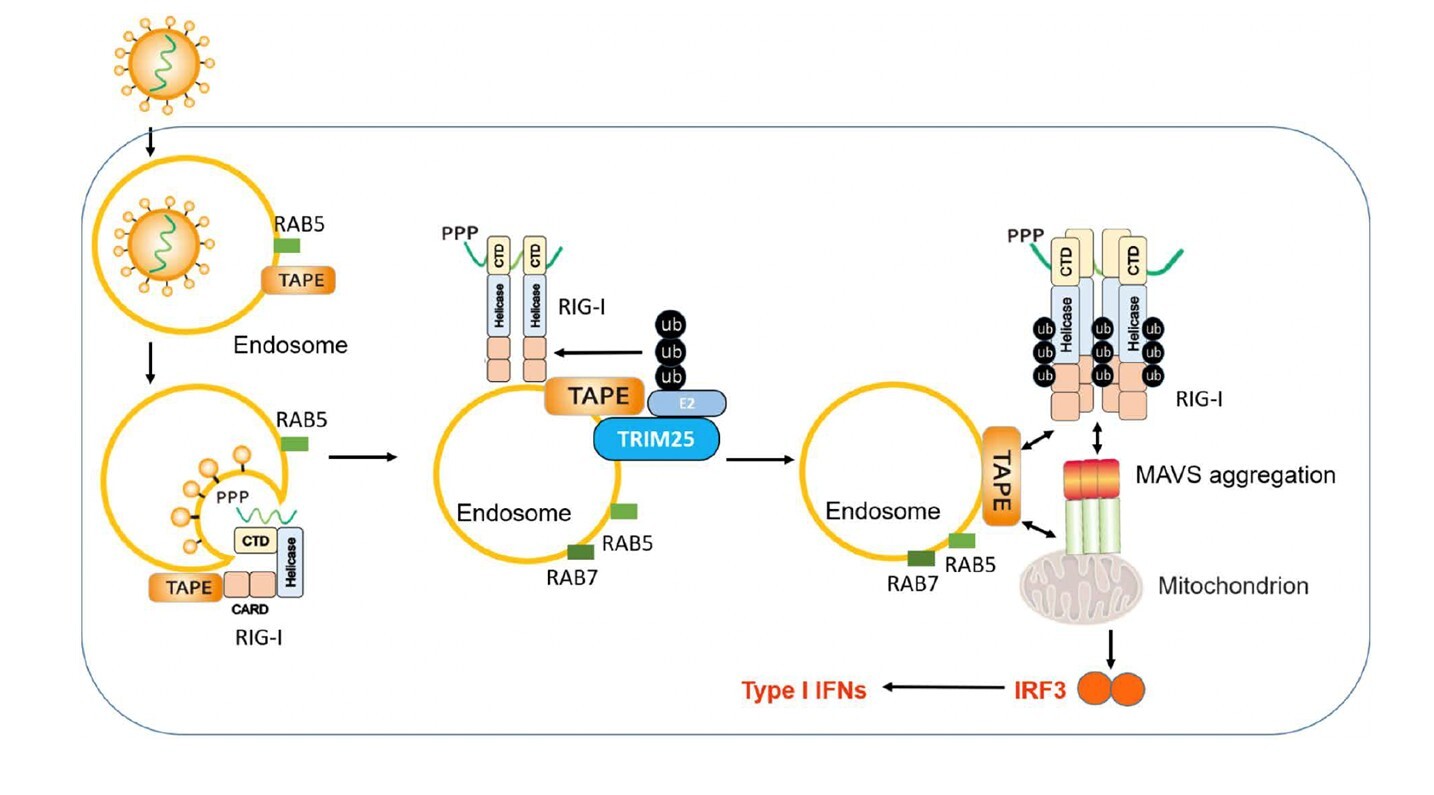
This novel RIG-I action mode by Ling’s work allows the host to detect viral infection and induce antiviral immunity much earlier than the previous idea.
This study entitled “Endosomes Serve as Signaling Platforms for RIG-I Ubiquitination and Activation” has been published in Science Advances, a prestigious journal under the Science group.






















